-
Telephone:400-882-7987
-
wechat:15523376158wechat:15950040099

Abstract: In the vast ocean of biomedical research and development, the selection of specific candidate molecules, off-target risk assessment and expensive overseas testing have always been like the turbulent and frequent surging waves, troubling the hard-working scientific researchers sailing in the endless waves. But today, a new battleship has sailed out of this ocean - the domesticated membrane protein array technology, which is not only the crystallization of China's independent innovation, but also the frigate that guards the development of the biomedical industry at home and abroad and waves.
and Retrogenix are leading international conies in the application of membrane protein array technology. Integral Molecular's Membrane Proteome Array technology has a comprehensive coverage of the protein library and a highly sensitive screening capability, which has made it widely recognized worldwide, according to the report. Integral Molecular has promoted over 2000+ monoclonal antibody research projects for more than 400 pharmaceutical companies and research institutes, and has published over 350+ papers [REF]. Retrogenix's Cell Miarray Technology provides detailed exploration of more than 6,500 full-length proteins, which makes it unique in the field of drug discovery and biotherapy candidate screening, and its test data submitted to the IND application regulatory authorities are widely recognized globally, according to the website. The success rate exceeds 95% [REF], and it has become a powerful propeller in the field of biological drug research and development.
The above data has shown the importance and recognition of membrane protein array, but the cumbersome overseas detection procedures and expensive detection, communication and other costs have been making domestic scientific researchers overwhelmed. Everyone is looking forward to the emergence of innovative, Membrane protein array technology.
Now, China's membrane protein array technology platform, built by Shouheng Biotechnology Co., Ltd. after 4 years, has finally come out. From the aspects of technical advancement and storage coverage, it can break hands with the two major international platforms. Let's take a look at it.
One of the core indicators for evaluating membrane protein array technology is the coverage of genes, as well as the standardization of detection and quality control methods. Firstly, the membrane protein granulation of Shou Heng Biological was formed by searching plasma membrane proteins in different databases, screening membrane proteins with high accuracy according to scores, and then screening functional membrane proteins by Shengxin analysis combined with AI prediction. After that, its cDNA was constructed into a vector containing marker genes to form a strain bank, and then a plasmid library was obtained by high-throughput plasmid extraction. Only after the performance of the plasmid reached the quality control standard could it be put into the library. The whole process and data were traceable, laying a stable and reliable foundation for the formation of human membrane protein array.
The Human membrane protein array platform is formed by transient transfer of 6000+ membrane proteins on HEK-293T to form a cell array. In the cell array, the optimal incubation concentration of the sample is first determined by positive control, and then the suspected binding protein is initially screened by high-throughput and high-sensitivity chemiluminescence detection. The suspected binding protein is identified under specific detection criteria and then expressed. 4 samples with different concentration gradients were incubated for flow cytometry to determine the binding relationship between proteins. Different quality control nodes were set up throughout the detection process. The same detection process and data can be traced, and the detection data report can be applied to IND declaration. The samples to be tested cover IgG, , ADC, VHH, CAR molecules, viruses, small molecules, peptides and so on under different protein labels.
Since the Human membrane protein array platform was officially put into use, Shouheng Biology has completed a large number of sample tests, accumulated the most objective and true data, and made many new discoveries and breakthrough results in the detection process. Here are several different case studies.
Case 1: Monoclonal antibody specificity analysis
In recent years, monoclonal antibody drugs, including antibody coupling drugs, have been widely used in the treatment of various diseases, but serious clinical side effects caused by off-target are not uncommon. In the process of antibody drug development, the closer the candidate molecules are to the terminal detection, the higher the cost of development failure, which is an indisputable fact. After off-target evaluation of many therapeutic antibodies already on the market, Shou Hengbio found that the off-target rate of Mabs was still as high as 16%. If it can be identified by high-sensitivity off-target analysis method in advance, combined with clinical manifestations, it is bound to guide the drug administration strategy in time, reduce the probability of side reactions, and improve the safety of drugs.
Specific case introduction: A humanized monoclonal antibody MAB for the treatment of malignant tumors, which can competitively bind EGFR, prevent the binding of epidermal growth factor and its receptor, inhibit the proliferation of tumor cells, and promote the apoptosis of tumor cells. For the treatment of EGFR-positive expression of stage III/IV nasopharyngeal carcinoma, pancreatic cancer patients, common side effects include fever, blood pressure drop, nausea, dizziness, dry mouth, rash and so on.
Specific data display:
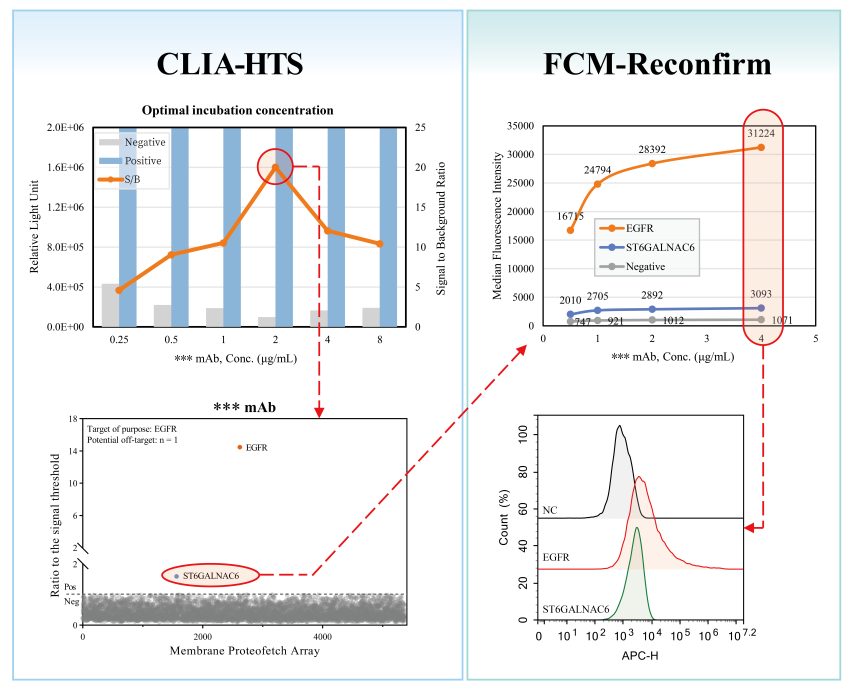
Detection phenomenon: The weak binding of ** monoclonal antibody to ST6GALNAC6 molecule was found through high-throughput preliminary screening. Then, the ** monoclonal antibody at 4 concentrations was incubated with cells overexpressing ST6GALNAC6 protein for flow detection, and it was found that the detection values at all concentrations were higher than the negative detection line.
Conclusion: Positive in the target, there is the risk of missing the target. The weakly bound ST6GALNAC6 molecule is sialyltransferase. Through the analysis of its molecular biology and clinical manifestations, the clinical side effects of "dry mouth" are suspected to be related to the off-target of this molecule.
Case 2: ScFv specificity analysis
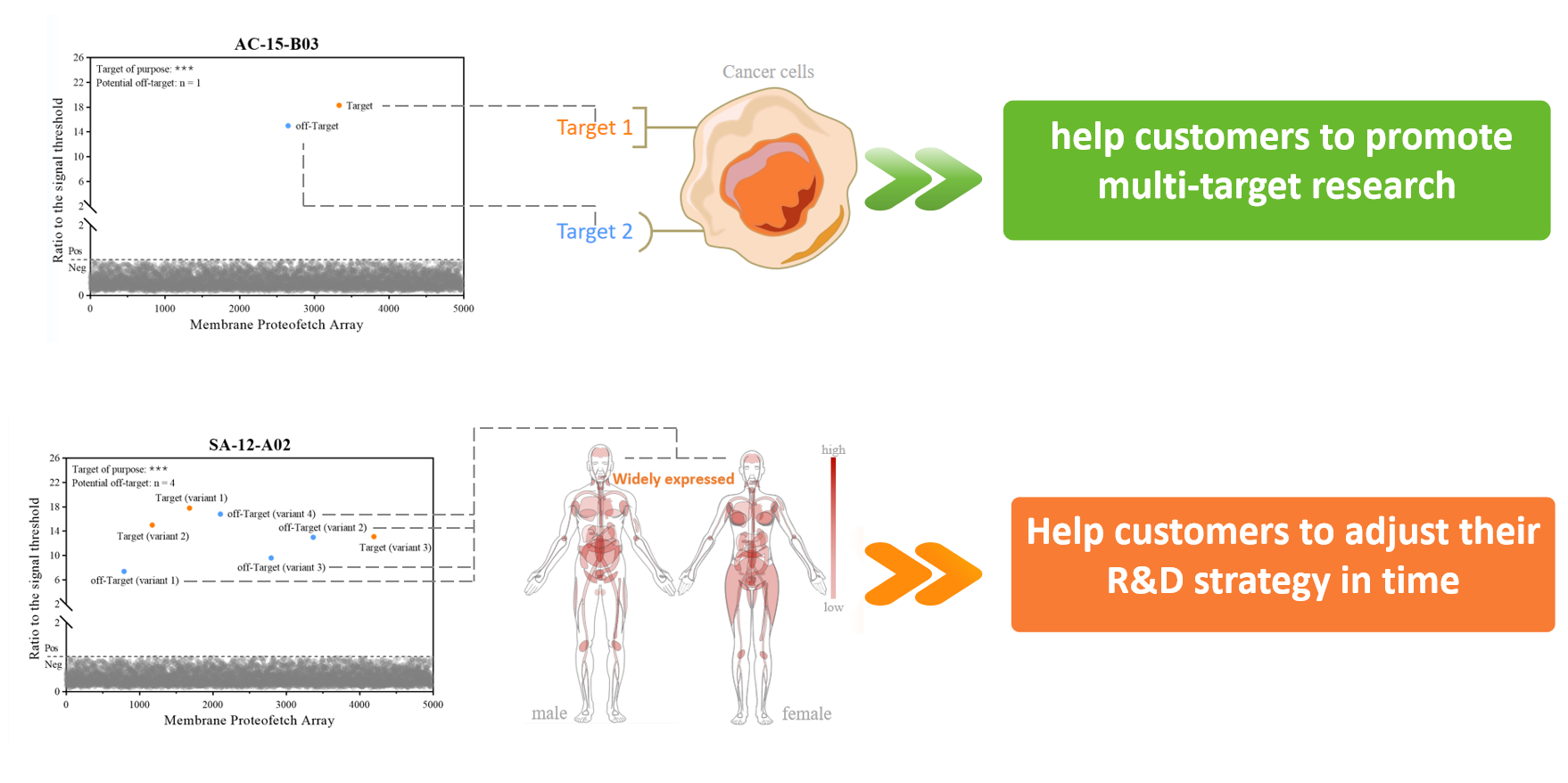
CAR-T therapy, as a "living drug" different from traditional drugs, continues to bring breakthrough surprises to the market in the field of tumor treatment. ScFv is one of the most critical components of CAR-T drugs, and its specificity determines the safety of CAR-T therapy. Off-target safety evaluation of CAR-T drugs is not only a necessary link in preclinical research but also a necessary data for IND application. The off-target evaluation of several cases of ScFv in CAR-T products under development found that the off-target samples not only missed to ubiquitous-expressed proteins, but also missed to other target antigens (two cases have been detected so far). Such data can help customers advance the layout of multi-target research and turn risks into opportunities.
In the face of technological competition from Britain and the United States, China's membrane protein array technology is not at a disadvantage. On the contrary, it shows its unique advantages in price, timeliness, communication and other aspects. We believe that the localization of membrane protein array technology, in the face of technological competition from Britain and the United States and other countries, China's human membrane protein array technology is not at a disadvantage. On the contrary, it shows its unique advantages in price, timeliness, communication and other aspects. It is believed that the localization of membrane protein array technology, like an advanced and trustworthy warship, guards the domestic and foreign biomedical enterprises to break through the waves, strive to move forward in the tide of biomedical research and development, and contribute China's wisdom and strength to the cause of human health.

