The membrane protein array technology is to express plasma membrane proteins in a natural state directly in cells
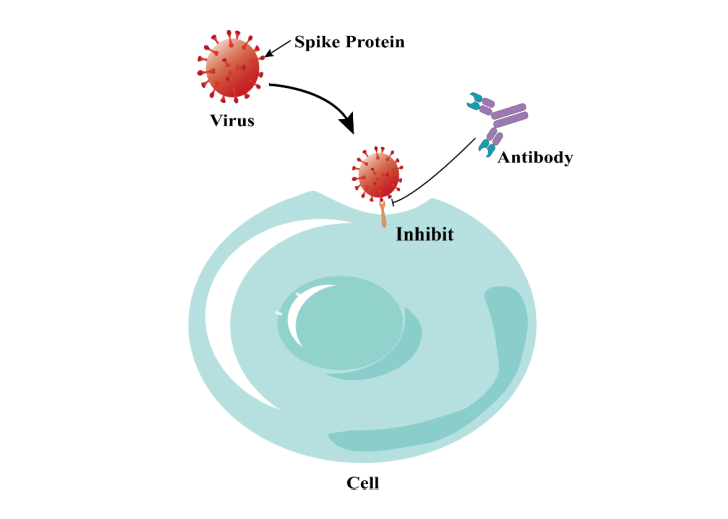
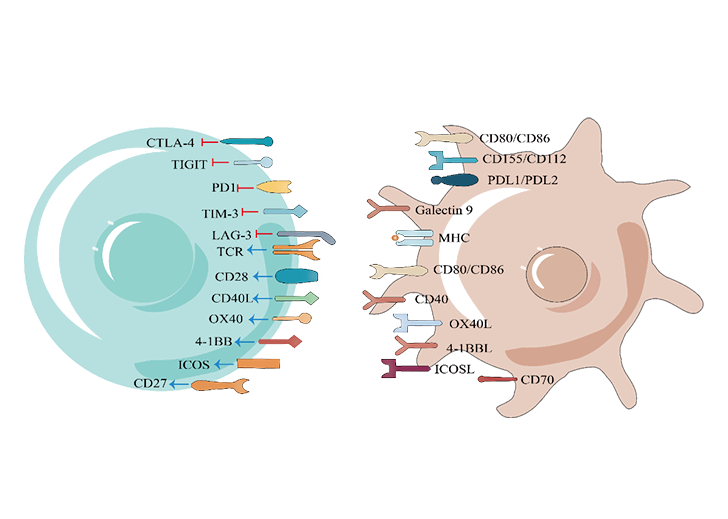
, keeping their structural integrity and natural post-translational modification, and on this basis, to form a cell array, which is used to identify the targeting specificity of antibodies, drugs or other ligands that bind to membrane proteins, and to provide a comprehensive cross-reaction assessment for the new regulatory requirements of IND declaration of CAR-T, antibodies and other drugs. At the same time, this technique can also be used in many fields, such as new target discovery, virus mechanism analysis, ligand-receptor de-orphaning .
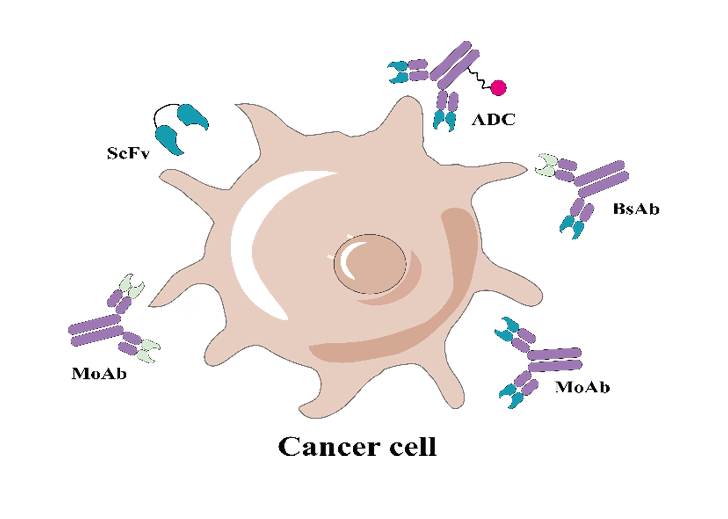
 Specificity analysis of ScFvAs a "live drug" different from traditional drugs, CAR-T therapy continues to bring breakthrough surprises to the market in the field of tumor therapy. ScFv is one of the most critical components of CAR-T, and its specificity determines the safety of CAR-T therapy. The off-target safety evaluation of CAR-T drugs is not only a necessary part of preclinical research but also a necessary data for IND application.More+
Specificity analysis of ScFvAs a "live drug" different from traditional drugs, CAR-T therapy continues to bring breakthrough surprises to the market in the field of tumor therapy. ScFv is one of the most critical components of CAR-T, and its specificity determines the safety of CAR-T therapy. The off-target safety evaluation of CAR-T drugs is not only a necessary part of preclinical research but also a necessary data for IND application.More+ Sepcificity analysis of monoclonal antibodyIn recent years, monoclonal antibody drugs including antibody coupling drugs have been widely used in the treatment of various diseases, but serious clinical side effects caused by missed targets are also common. in the process of antibody drug development, the closer the candidate molecules are to the terminal detection, the higher the price for the development failure. We evaluate the therapeutic antibodies on the market and find that the miss rate of Mabs is 16%....More+
Sepcificity analysis of monoclonal antibodyIn recent years, monoclonal antibody drugs including antibody coupling drugs have been widely used in the treatment of various diseases, but serious clinical side effects caused by missed targets are also common. in the process of antibody drug development, the closer the candidate molecules are to the terminal detection, the higher the price for the development failure. We evaluate the therapeutic antibodies on the market and find that the miss rate of Mabs is 16%....More+