-
Telephone:400-882-7987
-
wechat:15523376158wechat:15950040099

CAR-T (Chimeric Antigen Receptor T-Cell Immunotherapy), T cell immunotherapy with chimeric antigen receptor. This therapy is a new type of fine therapy that has been around for many years but has only been improved and used in clinical practice in recent years.
CAR-T(Chimeric Antigen Receptor T-Cell Immunotherapy), or chimeric antigen receptor T cellsImmunityTherapy. This therapy is a new type of cell therapy that has been around for many years but has only been improved and used in clinic in recent years. In acuteLeukaemiaAnd non-HodgkinLymphomaThe treatment has a significant effect and is considered to be the most promising.TumorOne of the treatments. Like all technologies, CAR-T technology has gone through a long process of evolution, and it is in this series of evolution that CAR-T technology is gradually becoming mature.
The key to this new treatment strategy is to identify artificial receptors called chimeric antigen receptors (chimeric antigen receptor, CAR) on target cells, and after genetic modification, patient T cells can express this CAR. In human clinical trials, scientists extracted some T cells from patients through a process similar to dialysis, and then genetically modified them in the laboratory to introduce the gene that encodes the CAR so that the T cells can express the new receptor. These genetically modified T cells proliferate in the laboratory and then infuse them back into the patient's body. These T cells use their expressed CAR receptors to bind to molecules on the surface of the target cells, and this binding triggers the production of an internal signal that then activates the T cells so strongly that they quickly destroy the target cells.
In recent years, CAR-T immunotherapy has not only been used to treat acute leukemia and non-leukemia.Hodgkin's lymphomaIn addition, after improvement, it is also used to treat solid tumors, autoimmune diseases, HIV infection, heart disease and other diseases, with a broader application space. Based on this, the editor makes an inventory of the latest progress made in CAR-T cell therapy in order to provide dinner for readers.
doi:10.1158/2159-8290.CD-22-1319
In a new study, Michel Sadelain Lab, M.D., MSK, found that destroying individual genes in CAR-T cells can make them stronger and more resistant to tumors. The results of the study were recently published in the journal Cancer Discovery. The paper is entitled "Disruption of SUV39H1-mediated H3K9 methylation sustains CAR T cell function".
In this paper, the authors demonstrate that disrupting the SUV39H1 gene has a knock-on effect: it restores the expression of several genes that help maintain T cell lifespan. They point out that this method improves the efficacy of CAR-T cells against a variety of cancers in mice. "if we can help CAR-T cells maintain their function by destroying a gene, it will have a wide range of therapeutic benefits," said Dr. Nayan Jain, co-lead author of the paper and a member of the Sadelain lab. "
"this new method requires fewer CAR-T cells, so it may be able to expand the range of patients eligible for this treatment," Dr. Jain said. It can also improve the efficacy of CAR-T cell therapy for each patient. "
"this new method can prolong the lifespan of CAR-T cells while maintaining their tumor-killing function, so we can use lower doses to treat patients, which may reduce the release of a cytokine," said Dr. Zeguo Zhao, co-lead author of the paper.SyndromesSerious side effects of cytokine release syndrome, CRS. "
2.Nature: shocked! CAR-T cell therapy may lead to reactivation of HHV-6 virus
doi:10.1038/s41586-023-06704-2
In a new study, researchers from Stanford University in the United States collected data from previous CAR-T cell therapy studies to explore the reactivation of chimeric antigen receptor (CAR) T cells (CAR-T) in humans in patients receiving CAR-T cell therapy.HerpesVirus type 6 (human herpesvirus 6, HHV-6). This study attempts to describe the characteristics of HHV-6 reactivation, especially in CAR-T cell therapy B.Cell lymphomaOr leukemia. They found that CAR-T cell therapy may cause CAR-T cells in patients to reactivate HHV-6. The results were published in the November 16, 2023 issue of Nature, entitled "Latent human herpesvirus 6 is reactivated in CAR T cells".
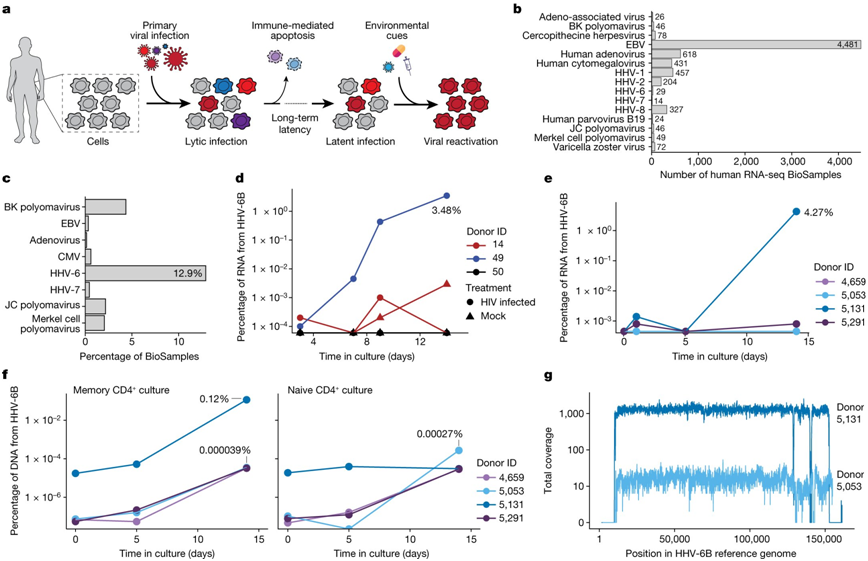
The picture is from Nature, 2023, doi:10.1038/s41586-023-06704-2.
The authors reanalyzed the scRNA-seq data sets of three groups of patients treated with autologous CAR-T cell products. Although no HHV-6 virus transcript was detected in CAR-T cell products before infusion, a significant increase in HHV-6+ cells was observed in the samples after infusion.
The authors found that 28 CAR-T cells in the sample after infusion expressed HHV-6B transcripts, of which 13 CAR-T cells were similar to the rare HHV-6 "superexpression (super-expressor)" cells in allogeneic CAR-T cells. Detailed time course analysis of two patients with HHV-6 hyperexpression showed that HHV-6+ cells appeared one week after treatment, which was consistent with the clinical symptoms of immune effect-associated neurotoxic syndrome. Symptoms such as insanity and neurocognitive iirment are consistent with the presence of HHV-6 in the blood, but not cause and effect in all patients.
These findings suggest a link between T cell activation, proliferation, and culture duration and HHV-6 reactivation, highlighting issues that need to be further considered in the development and monitoring of CAR-T cell therapy.
3.Nat Immunol: using modified CAR-T cells to improve the efficacy of cancer immunotherapy
doi:10.1038/s41590-023-01658-z
In a new study, researchers from the University of Freiburg in Germany can now significantly improve the effectiveness of treatment by preventing CAR-T cells from failing in preclinical animal models. The results of the study were published online in the journal Nature Immunology on November 6, 2023. The paper is entitled "Harnessing CD3 diversity to optimize CAR T cells".
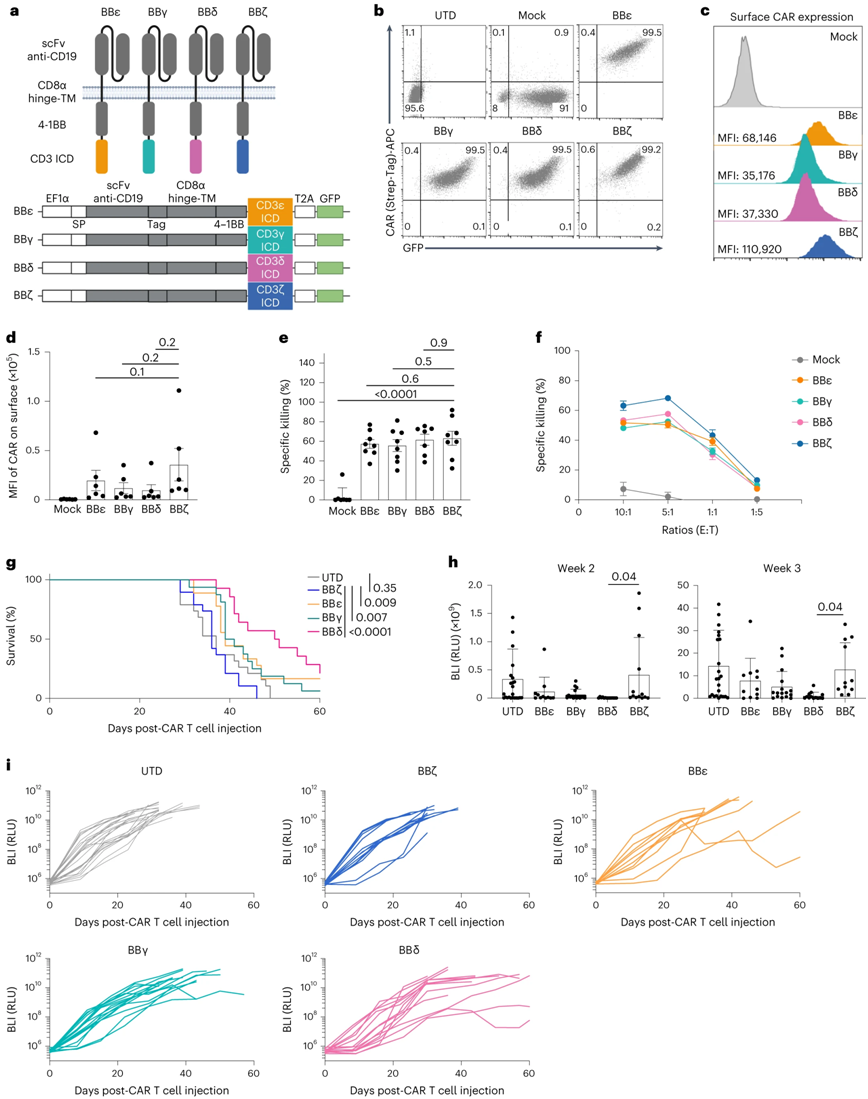
CAR with CD3δ / ε / γ cytoplasmic domain is better than CAR with perylene chain. The picture is from Nature Immunology, 2023, doi:10.1038/s41590-023,01658murz.
CAR functions like a sensing protein through which T cells recognizeCancer cellThe characteristics of the surface. Synthetic CAR contains some components of natural T cell receptor (TCR), but its structure has been greatly simplified. CAR has only one of four different subunits of TCR that transmit signals that trigger unmodified T cells to activate the immune response.
Dr Susana Minguet, author of the paper's correspondence and from the University of Freiburg, explained, "CAR approved by drug regulators uses the so-called Zeta chain, which triggers a particularly strong activation of T cells once CAR binds to the surface of cancer cells. Whether the other three signal transduction chains of T cell receptors-γ, δ and ε-can also be used in CAR has not been studied. "
In the new study, the authors created four types of CAR-T cells, each of which expressed a CAR carrying one of the four signal transduction subunits and tested them in a mouse model of leukemia. Schamel explained, "surprisingly, the antitumor effect of the curium chain used by clinical CAR-T cells is lower than that of the other three domains: γ, δ and ε chains. These domains have a better effect on the elimination of cancer cells in the leukemia model. "
4.Nat Commun: revealing mitochondriaFunctional disorderIt leads to T cell failure and is expected to improve CAR-T cell therapy.
doi:10.1038/s41467-023-42634-3
In the process of fighting cancer and infection, the immune system often leads to T cell failure: in the process, T cells gradually lose function, thus disrupting their response to cancer and infection. The molecular mechanism that controls this loss of function has not been fully unraveled.
In a new study, Martin Vaeth, an immunologist at the University of W ü rzburg in Germany, and his team found a possible explanation for this: the process of T cell failure is largely affected by "cell energy factory" mitochondria. The relevant research results were published in the journal Nature Communications on October 27th, 2023. The paper is entitled "Mitochondrial dysfunction promotes the transition of precursor to terminally exhausted T cells through HIF-1 α-mediated glycolytic reprogramming".
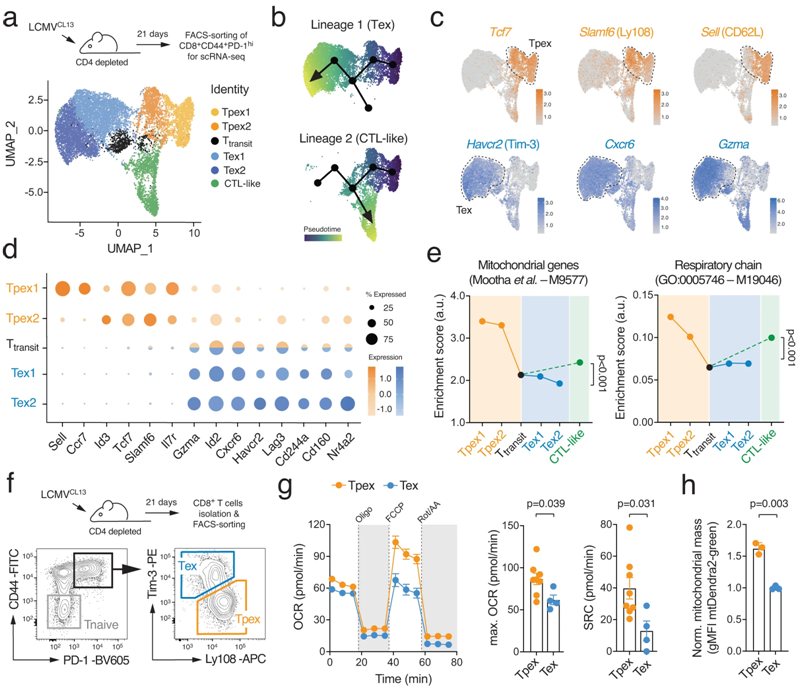
T cell failure is characterized by metabolic reprogramming. Picture from Nature Communications, 2023, doi:10.1038/s41467-023-42634-3.
When mitochondrial respiration fails, a series of responses are triggered, eventually leading to genetic and metabolic reprogramming of T cells, a process that drives T cell failure. However, this "exhaustion" of T cells can be offset-through drug or genetic optimization of cell metabolism can prolong the life span of T cells and enhance their function. For example, this can be achieved by overexpressing a mitochondrial phosphate transporter that promotes the production of the energy-supplying molecule adenosine triphosphate (ATP).
"it is generally believed that changes in mitochondrial (energy) metabolism are the result of T cell failure," Vaeth said. To prove that mitochondrial dysfunction is the real cause of T cell failure, his team developed a new genetic model. It turns off the phosphate transporter Slc25A3, paralyzing mitochondrial respiration in T cells.
As a result, these T cells are forced to switch to other metabolic pathways, mainly aerobic glycolysis, to meet their bioenergy needs in the form of adenosine triphosphate. However, this metabolic adaptation can lead to an increase in the production of reactive oxygen species in T cells. The increase of the level of oxygen free radicals will prevent the degradation of transcription factor HIF-1 α. The accumulation of HIF-1 α protein will lead to T cell genetic and metabolic reprogramming, thus accelerating T cell failure.
"this HIF-1 α-dependent control of T cell failure is not previously known," Vaeth explained. It represents a key regulatory pathway between mitochondrial respiration and T cell function and is a 'metabolic checkpoint' in the process of T cell failure. "
doi:10.1002/btpr.3388
Immunotherapy is a promising therapy that uses the power of the body's immune response to target cancer cells. A new tool for rapidly culturing immune cells that kill cancer may promote the availability of immunotherapy.
In a new study, researchers from Washington State University in the United States have developed a mini-refrigerator-sized bioreactor that can produce immune cells called T cells at 95% of the maximum growth rate-about 30% faster than prior art. They developed the technique using T cells from cattle and expect it to play a similar role in human cells. The results of the study were recently published in the journal Biotechnology Progress. The paper is entitled "Development of a centrifugal bioreactor for rapid expansion of CD8 cytotoxic T cells for use in cancer immunotherapy".

Kitana Kaiphanliam, lead author and postdoctoral fellow at Washington State University, said, "the growing manufacturing demand for this immunotherapy cannot be met, so there are gaps in biomanufacturing solutions that need to be filled. In the final analysis, they need to be upgraded so that they can be used by more people. "
The bioreactor uses centrifugal force to act on growing T cells while suspending them in dense clouds and constantly bathing in inward flowing media containing nutrients. The prototype is the result of 40 years of centrifugal bioreactor design research led by Bernie Van Wie, a professor of chemical engineering at Washington State University.
The latest prototype is also in a sterile cabinet. "it's like a biosafety cabinet," Kaiphanliam said. It can be used without clean production facilities or without easy access to clean production facilities, so these cell-based therapies can be popularized. "
6.Nature: found that trans isooleic acid in beef and dairy products increases the ability of CD8+ T cells to infiltrate tumors and kill cancer cells.
doi:10.1038/s41586-023-06749-3
In a new study, researchers from the University of Chicago, St. Jude's Children's Research Hospital and Emory University found that trans-vaccenic acid (TVA), a long-chain fatty acid found in meat and dairy products from grazing animals such as cattle and sheep, increases the ability of CD8+ T cells to infiltrate tumors and kill cancer cells. They also point out that patients with higher levels of TVA circulation in their blood respond better to immunotherapy, suggesting that TVA may be used as a nutritional supplement to assist clinical treatment of cancer. The results of the study were published online in the journal Nature on November 22, 2023. The paper is entitled "Trans-vaccenic acid reprograms CD8+ T cells and anti-tumour immunity".
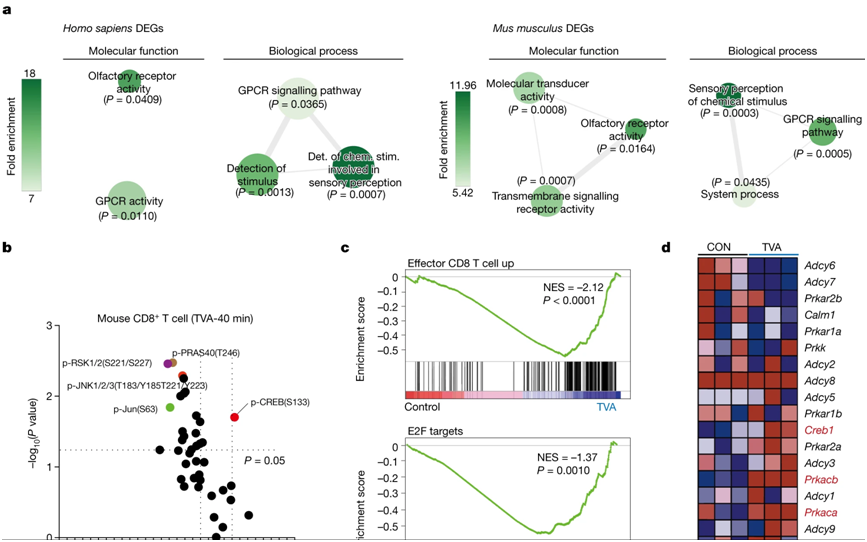
The picture is from Nature, 2023, doi:10.1038/s41586-023-06749-3.
Dr. Jing Chen, co-author of the paper and professor of medicine at the University of Chicago, said: "there are many studies trying to unravel the link between diet and human health, but because people eat so many kinds of food, it is very difficult to understand the underlying mechanism. But if we focus only on nutrients and metabolites in food, we can begin to understand how they affect physiology and pathology. By focusing on nutrients that activate T cell responses, we have found a way to actually enhance resistance by activating an important immune pathway.Tumor immunityReactive nutrients. "
Chen Labs focuses on understanding how circulating metabolites, nutrients and other molecules in the blood affect cancer development and response to cancer treatment. In the new study, two postdocs-- Dr. Hao Fan and Dr. Siyuan Xia-- created a library of "blood nutrients" compounds from a database of about 700 known metabolites from food. They screened the compounds in the new compound library to determine whether they could affect the anti-tumor immune response by activating CD8+ T cells, a class of immune cells critical to killing cancer cells or cells infected by viruses.
They evaluated the first six candidate compounds in human and mouse cells and found that TVA performed best. TVA is the highest content of trans fatty acid in breast milk, but the human body cannot produce it on its own. Only about 20% of TVA is broken down into other by-products, with the remaining 80% circulating in the bloodstream. "this means that it must have other functions, so we started to do more research on it," Chen said.
The authors then conducted a series of experiments with cells of different tumor types and mouse models. TVA rich diet significantly reduced the number of mice fed TVA compared to mice fed a control dietmelanintumors andcolon cancerTumor growth potential of cells. A diet rich in TVA also enhanced the ability of CD8 + T cells to infiltrate tumors.
These additional assays, performed jointly by Chen's lab and He's lab, showed that TVA inactivates a receptor on the cell surface called GPR43, which is normally activated by short-chain fatty acids frequently produced by gut mibiota. TVA suppresses these short-chain fatty acids and activates a cellular signaling process called the CREB pathway, which is involved in a variety of cell growth, survival and differentiation functions. They also found that mouse models in which the GPR43 receptor in CD8 + T cells was completely eliminated also failed to improve anti-tumor ability.
Finally, in collaboration with Dr. Justin Kline, professor of medicine at the University of Chicago, they analyzed blood samples from lymphoma patients treated with CAR-T cell immunotherapy. They observed that patients with higher TVA levels tended to respond better to treatment than patients with lower TVA levels. They also teamed up with medical professor Dr. Wendy Stock to test leukemia cell lines and found that TVA enhanced the ability of immunotherapy drugs to kill leukemia cells.
doi:10.1038/s41586-023-06733-x
T cells are key components of the immune system involved in killing cancer. Tumors produce signals that shut down these T cells, in part by forcing them to differentiate (mature) into a dysfunctional state known as exhaustion.
In a new study, researchers from St. Jude Children's Research Hospital in the United States have comprehensively studied transcription factors involved in the differentiation state of T cells in cancer. They then used this information to enhance anticancer activity in preclinical models by promoting or preventing T cell differentiation. These findings have important implications for cancer immunotherapy. The results were published online November 15, 2023, in Nature under the title "Single-cell CRISPR screens in vivo map T cell fate regulators in cancer."
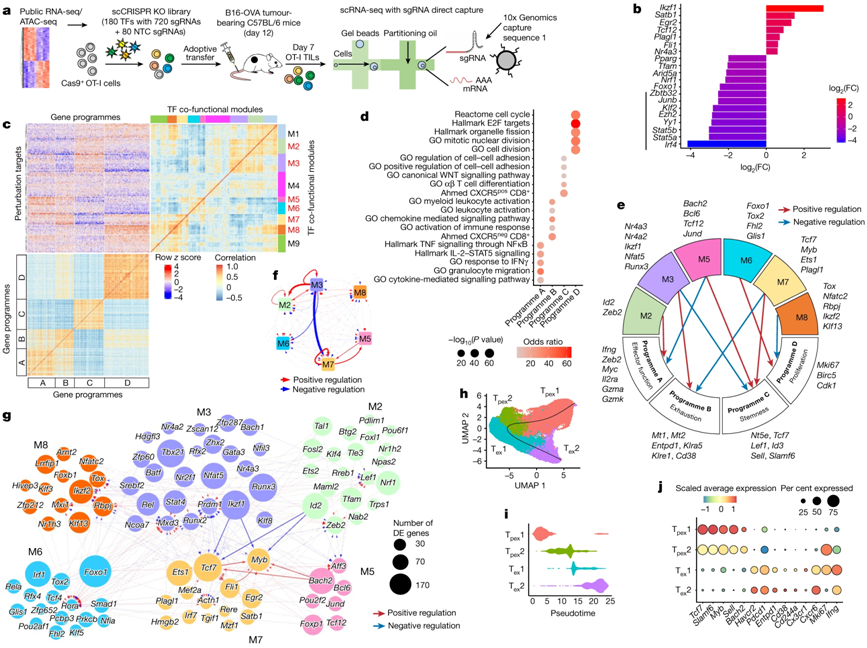
Image from Nature, 2023, doi:10.1038/s41586-023-06733-x.
T cells used in adoptive cell therapy (ACT) are used to target cancer cells. Chimeric antigen receptor (CAR) T (CAR-T) cell therapy as one of ACT has clinical efficacy for blood cancer, but has poor efficacy for solid tumor. This difference in efficacy is partly due to the fact that tumors promote T cell exhaustion, which makes T cells less effective at actively killing cancer cells. The authors found that they could precisely interrupt the T-cell differentiation process, resulting in improved anti-tumor efficacy.
Hongbo Chi, PhD, Department of Immunology, St. Jude Children's Research Hospital, said: "T cells are the cornerstone of tumor immunotherapy, and we have found a new way to reprogram T cells to make them more effective." We can push them to a specialized state where they become more powerful tumor killer cells."
8.Nat Commun: Developing potent gamma delta T cells, promising universal cancer immunotherapy
doi:10.1038/s41467-023-42619-2
"Off-the-shelf" cell therapy, also known as allogenic cell therapy, uses immune cells from healthy donors rather than patients. This approach could provide cell therapies such as chimeric antigen receptor (CAR) T cell therapy to more patients in a more timely manner, which is one of the major obstacles to providing these life-saving therapies to patients.
In a new study, researchers from the University of California, Los Angeles, have developed a new way to engineer more powerful immune cells that could potentially be used in "off-the-shelf" cell therapies to treat challenging cancers. This new approach is a key step toward developing more effective cell therapies that are cheaper, have shorter wait times, and can be mass-produced and shipped to hospitals around the world, making CAR-T cell therapies cheaper and more widely accepted by patients. Unlocking the potential of allogeneic Vδ2 T cells for ovarian cancer therapy through CD16 biomarker selection and CAR/IL-15 engineering.
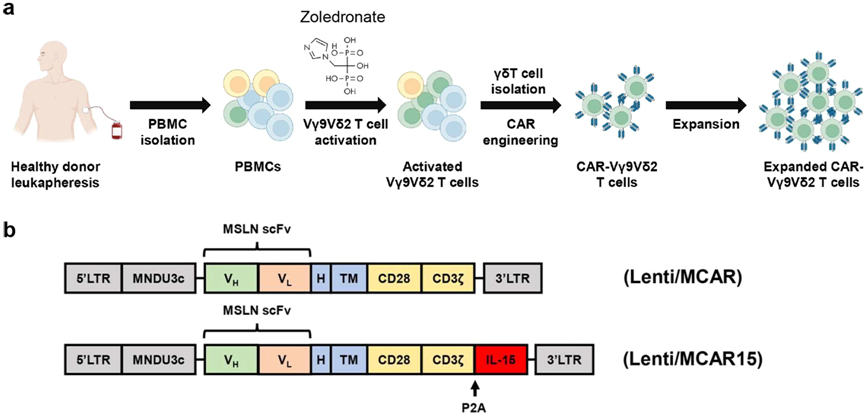
CD16-overexpressing Vδ2 T cells genetically engineered to express CAR/IL-15 maintain both expansion and memory. Image from Nature Communications, 2023, doi:10.1038/s41467-023-42619-2.
"time is often critical when treating patients with advanced cancer," said Lili Yang, author of the paper and associate professor of microbiology, immunology and molecular genetics at the University of California, Los Angeles. At present, this kind of treatment needs to be tailored to the specific situation of the patient. We have to extract immune cells called T cells from the patient, genetically modify these cells, and then infuse them back into the patient's body. This process can take weeks to months, and treating each patient can cost hundreds of thousands of dollars. "
In the new study, Yang and her team focused on gamma delta T cells (gamma delta T cell), immune cells known for targeting a variety of cancers, including solid tumors, but not causing graft-versus-host disease, a common complication of allogeneic cell therapy.
Although γ δ T cell-based therapies have been previously studied, the clinical success rates of these therapies are limited due to the variability and transient persistence of donors and the ability of cancer cells to evade or evade human immune responses.
However, Yang and her team found that donor gamma δ T cells that highly expressed CD16 surface markers were more capable of killing cancer cells. "these γ δ T cells with high expression of CD16 show unique characteristics and improve their ability to recognize tumors," Yang said. They show higher levels of effector molecules and have the ability to produce antibody-dependent cytotoxicity to cancer cells. We found that their anticancer properties can be improved by using CD16 as a biomarker for donor selection. "
9.Cell: new research shows that CAAR-T cell therapy is promising for autoimmune encephalitis
doi:10.1016/j.cell.2023.10.001
In a new study, researchers from the German Research Centre for Neurodegenerative Diseases and the Charlotte School of Medicine in Berlin have pioneered a new treatment for the most common autoimmune encephalitis called NMDA receptor encephalitis (NMDA receptor encephalitis). By reprogramming leukocytes symmetrically to eliminate pathogenic cells, the accuracy and efficiency of this method have reached a new level. It has been successful in laboratory research and human clinical trials are being planned. The results were published online in the journal Cell on November 1, 2023. The paper is entitled "Chimeric autoantibody receptor T cells deplete NMDA receptor-specific B cells".
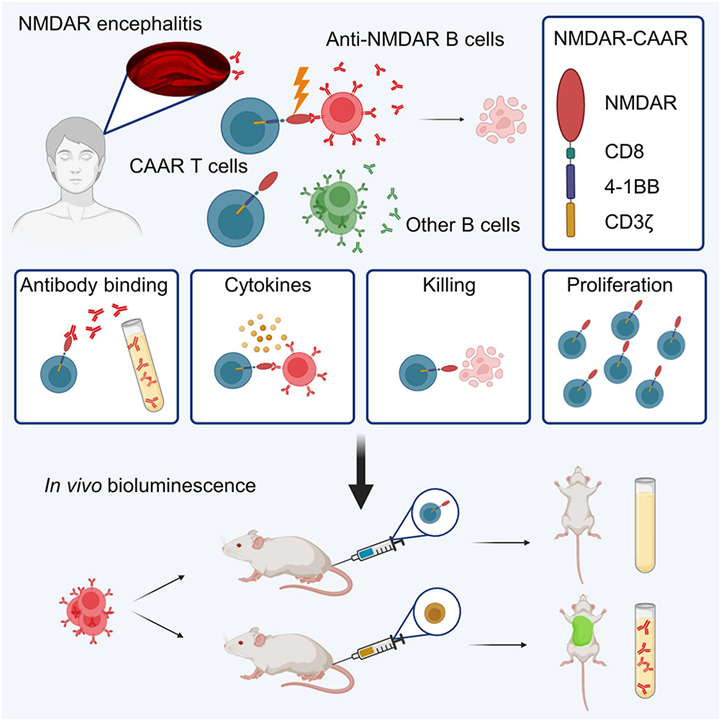
Picture from Cell, 2023, doi:10.1016/j.cell.2023.10.001.
NMDA receptor encephalitis is one of the most common brain diseases caused by antibodies. In this disease, anti-NMDA receptor antibodies suddenly attack the brain, which in turn attacks the patient's own body. Professor Harald Pr ü ss, co-author of the paper, said, "in preclinical trials, we successfully selectively shut down the B cells that form these mistargeted antibodies (anti-NMDA receptor antibodies)."
The authors designed special chimeric autoantibody receptors (chimeric autoantibody receptor, CAAR) T cells (CAAR-T) for injection into patients. These programmed CAAR-T cells can recognize and eliminate B cells that produce antibodies against NMDA receptors with high precision. In the mouse model, this innovative method shows its accuracy.
Once these CAAR-T cells re-enter the body, they specifically attack B cells that produce antibodies against NMDA receptors. The surface shape of these B cells allows CAAR-T cells to precisely dock with them and kill them. Importantly, B cells that produce other antibodies and therefore have different surface shapes are not affected. (Biological valleyBioon.com)

