-
Telephone:400-882-7987
-
wechat:15523376158wechat:15950040099

Antibody-coupled drugs (Antibody-drug conjugates, ADC) combine the specificity of monoclonal antibodies with the efficacy of highly cytotoxic drugs, and target their payloads to the tumor site first, thus potentially reducing the severity of side effects.
Antibody-coupled drugs (Antibody-drug conjugates, ADC) combine the specificity of monoclonal antibodies with the efficacy of highly cytotoxic drugs, and target their payloads at the tumor site first, thus potentially reducing the severity of side effects, which is called the "magic bullet" of cancer therapy.
ADC is increasingly used in combination with other drugs, including for first-line cancer treatment. As the technology for producing these complex treatments matures, more ADC drugs are approved or in late clinical trials. The diversification of antigenic targets and bioactive payloads is rapidly expanding the range of tumor indications of ADC. In addition, new forms of carrier proteins and warheads targeting tumor mienvironment are expected to improve the intratumoral distribution or activation of ADC, thus improving its anticancer activity against refractory tumors. However, toxicity remains a key issue in the development of these drugs. Better understanding andAdministration and ManagementADC-related toxicity is very important for its further optimization.
A review published on Nature Reviews Drug Discovery on June 12 details the latest progress and challenges in ADC development in cancer treatment. Some of the main points of this excerpt are shared with you.

An overview of ADC's listing
ADC is a complex therapeutic drug consisting of three key components, including antibodies, connectors, and payloads (figure 1). Optimizing each of these components produces an improved ADC.
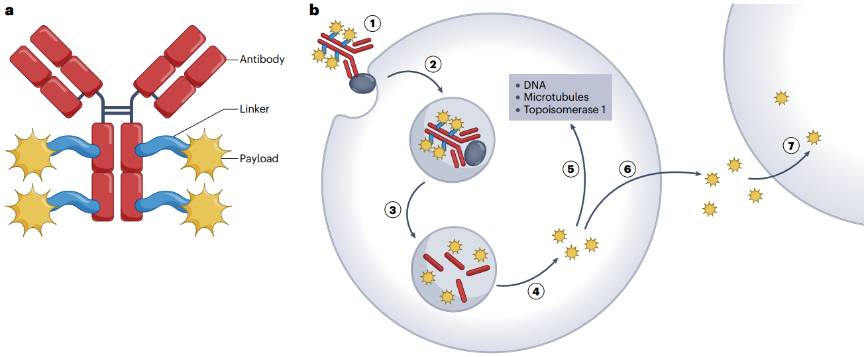
Figure 1 | structure and mechanism of traditional ADC
At present, more than a dozen ADC have been approved to be listed in the world. Among them, 6 kinds of antigens targeting hematological malignant tumors: CD33, CD30, CD22, CD79b, BCMA and CD19;7 target 5 different antigens: HER2, nectin-4, TROP2, tissue factor (TF) and folate receptor α (FR α).
Solid tumors provide a broad opportunity for the development of ADC because they are more common than hematological malignancies, have relatively few treatment options in advanced or metastatic cases, and are rarely passed.ImmunityThe cure. In this regard, the nectin-4 targeted ventozumab (Padcev) approved in 2019 for the treatment of locally advanced or metastatic urothelial malignant tumors and the triple negative treatment approved in 2020breast cancerThe TROP2 targeting of Gosatuzumab (Todavi) is significant because they provide new options for a limited selection of diseases.

Figure 2 | main features of approved ADC
In addition, as of December 2022, ADC drug candidate trastuzumab duocarmazine is under regulatory review in the United States (PDUFA date is May 12, 2023). The other two types of ADC datopotamab deruxtecan and tusamitamab ravtansine listing applications are likely to be filed in 2023.
Interestingly, all approved ADC drugs are based on cysteine coupling (DAR 4 to 8) or random lysine coupling (DAR 2). Although site-specific coupling has shown promising results in vitro and in vivo, it has not been successful in clinic so far. Many of these ADC failed in clinical trials from phase I to II. Only 2 of the 21 ADC in later trials [ARX788 (Ambrx) and pivekimab sunirine/IMGN632 (ImmunoGen)] are based on site-specific coupling. In addition, most approved (11amp 13) and current late clinical stage (19Univer 21) ADC have cleavable connectors and non-polar payloads, resulting in bystander killing effects.
Address the limitations of ADC
As with most drugs, the interruption in the development of ADC is usually due to a lack of efficacy, safety issues, commercial considerations, or a combination of these factors. Of the 97 ADC that entered clinical trials and terminated since 2000, most (81; 84%) terminated in Phase I or I/II, only 12 and 4 terminated in Phase II and III, respectively; and most (67%) contained tubulin binding payloads, 24% contained DNA targeted agents (including two kazithromycin payloads and 21 PBD derivatives), and 3% contained topoisomerase 1 inhibitors. Many (80 per cent) target tumor antigens that are not representative of approved therapeutic targets, 18 are targeted at validated targets; in addition, 32 ADC were interrupted due to lack of efficacy, 32 were interrupted due to safety problems, and 29 were interrupted due to commercial concerns; several of them were developed for hematoma, most of them for solid tumors.
What lessons can be learned from these terminated projects? The lack of antineoplastic activity at the maximum tolerated dose seems to be the main cause of termination. In addition, unacceptable toxicity remains a major obstacle to the development of new ADC drugs. Better prediction of expected serious adverse events would be another way to reduce the premature termination of drug development. For example, ADC HTK288 targeting cadherin-6 is associated with accidental central nervous system toxicity, while ADC LOP628 targeting tyrosine kinase receptor KIT is associated with unexpected severe hypersensitivity. As the development of cisplatin and paclitaxel has proved in the past, managing the toxicity of a new compound is often a long-term effort.
In general, it is difficult to select the right combination of appropriate target antigen, active connector-payload, appropriate Dar value and appropriate tumor indications. With the rapid growth of the number of validated target antigens and the diversification of payloads, it is expected that at least some antigen-targeted terminated ADC need to be further developed.
1. Diversification of payload
Prior to the approval of Detrozumab in 2019, the payloads of marketed ADC can be divided into two categories: tubulin binders and DNA targeting agents. Now, many other drug molecules have been evaluated as potential payloads.
Auristatin derivatives interfere with the kinetics of tubulin polymerization and play a powerful role by destroying the formation of mitotic spindles, leading to mitotic arrest and cell death. ADC with Auristatin is currently the largest ADC family (figure 3).
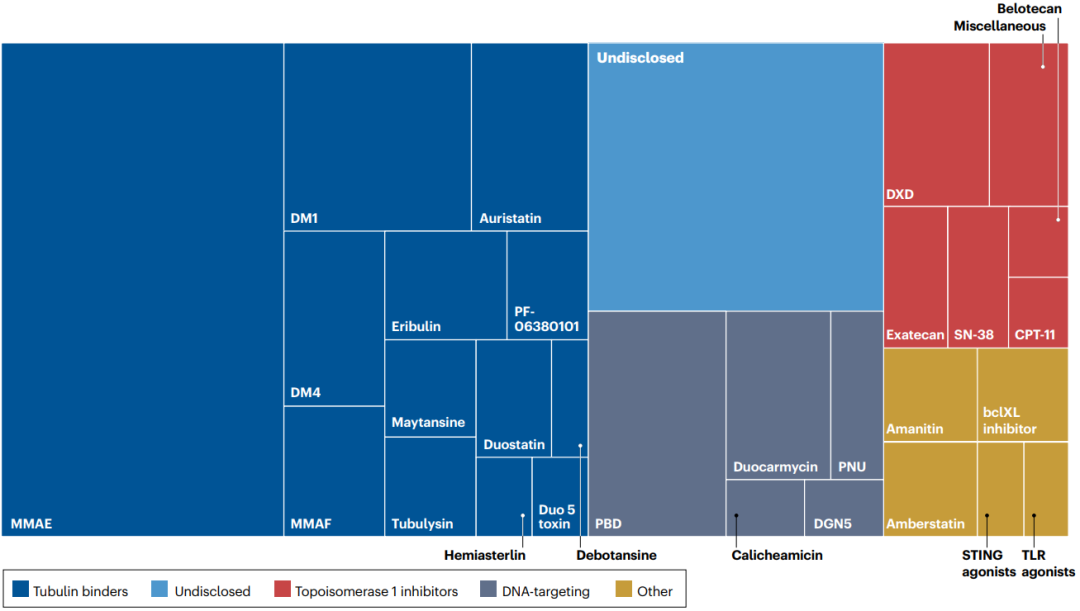
Figure 3 | diversification of payloads in the ADC pipeline
The second most representative payload is the DNA targeting agent, which chemically modifies DNA to prevent cell replication. Calicheamicin is a powerful DNA damaging agent, which causes double-stranded DNA (dsDNA) to break through free radical mechanism, and is also the payload used in two approved ADC. PBD dimer is an alkylating agent of cross-linked dsDNA and is one of the most effective cytotoxic agents identified.
Until recently, most ADC under development used potent cytotoxins as "warheads". This is partly due to the observation that high DAR values are associated with less favorable pharmacokinetic properties, so a more effective payload is preferred, resulting in effective ADC at Dar values of 2 to 4. Recently approved T-DXd and Gosatuzumab with a Dar value of about 8 indicate that it is possible to connect a larger number of cytotoxic molecules to antibodies without affecting solubility, aggregation tendency or pharmacokinetic properties. This leads to a paradigm shift, which makes it possible to study low-potency compounds with different mechanisms as payloads for ADC.
Given that not all types of tumors are sensitive to a given type of payload, payload diversification is essential for expanding ADC indications. Over the past 20 years, we have witnessed the successful development of ADC, and these families have strong members. With regard to the development of payloads, three observations can be made: first, not all frequently used families of cytotoxic agents have been successfully used as ADC payloads. In particular, attempts to develop nucleoside analogues and antimetabolites as payloads have failed. Second, ADC that has not yet been approved contains payloads that have a cytotoxic mechanism that is very different from that of conventional chemotherapy. Finally, among a large number of new drugs with original mechanisms evaluated in clinical trials, including kinase inhibitors and molecules targeting various processes in tumor cells, a large number of drugs failed because of poor safety. However, some of these drugs may be potential candidates for ADC payloads. Heidelberg Pharma has brought an example of this type of development to the clinic, even with α-muscarine derivatives as a new ADC payload.
Given the complexity of ADC, payload diversification may be seen as a risky effort. If the payload binds to antibodies from proven targets such as HER2, the results may be very promising, as in the case of detrazumab. However, it is difficult to estimate how many second or later generations of ADC will be successfully developed for a given target. On the contrary, exploring new payloads associated with unverified targets increases the risk of failure and complicates the assessment of its root causes.
2. Attach importance to toxicity
According to the side effects usually associated with the type of payload considered, the toxicity associated with ADC administration can be classified as "expected" and "accidental".
Expected toxicity: for example, MMAE induces peripheral neuropathy, which is a typical side effect of tubulin binders. Myelotoxicity is a common complication of most cytotoxic chemotherapeutic drugs, especially DNA targeting drugs.
Accidental toxicity: for example, although MMAE-based ADC is usually not associated with ocular toxicity, MMAF is associated with corneal toxicity when approved for use with ADC (such as belantamab mafodotin), with up to 72% of patients showing epithelial changes. In addition, as ADC is increasingly included in joint programmes, other unexpected toxicity may be observed. In Hodgkin.LymphomaAmong patients, vetuximab (BV) in combination with standard care regimens containing bleomycin resulted in severe pulmonary toxicity in 44% of patients, but not in the non-bleomycin group. The potential mechanisms of these different toxicities are not fully understood, which may involve Fc-mediated ADC internalization, payload release in normal tissues, off-tumour expression of target antigens, non-specific uptake of ADC through pinocytosis or similar cellular processes, enzymatic release of payloads in systems or specific normal tissue environments, or Fc-mediated inflammation in the case of payload-mediated tissue damage.
As ADC will increasingly be used as first-line drugs or adjuvants, its long-term safety and reversibility of side effects will become more and more important. An important problem is the mutagenic effect that may be produced by some payloads, especially the DNA targeting agents. Peripheral neuropathy may be irreversible in some patients, which emphasizes the need for adaptive drug administration and close follow-up for patients at risk. Another key issue is to determine whether certain patients require specific drug delivery arrangements based on variables such as age, sex, type and number of previous treatments, complications or genetic characteristics.
3. Overcoming drug resistance
Given the series of steps required for the successful cytotoxicity of ADC, the underlying mechanism of ADC resistance may be complex. The drug resistance of ADC can be observed under the conditions of decreased antigen binding and / or antibody / antigen internalization, decreased intracellular concentration of payload, change of payload target, change of apoptosis mechanism and so on.
Several therapeutic interventions can improve the efficacy of ADC in preclinical model. One possibility is to strengthen the internalization of ADC, the mechanism of which is still unclear. The overexpression of caveolin-1 related to lipid rafts increased the internalization of T-DM1. In cases where drug resistance has been established, a second type of payload can be used.
Heterogeneity of antigen expression is a classical mechanism of antibody-based treatment failure. Preclinical models have confirmed that the distribution of antibodies in tumors depends on the expression of target antigens. The bystander effect now provided by most ADC (such as T-DXd), including the release of unbound payloads in the tumor microenvironment and the potential uptake by neighboring tumor cells independent of their antigen spectrum, is a powerful means to combat intra-tumor antigen heterogeneity, because the payload can reach tumor cells with low target antigen levels.
In addition, the mode of administration of ADC in patients is also expected to have a strong ict on the occurrence of drug resistance. In early trials, ADC was administered mainly as a single drug, which led to the selection of drug-resistant tumor populations. At present, many combination schemes are being explored, including traditional cytotoxic chemotherapy and other targeted drugs. Treatment ordering may also be an important parameter.
4. Combination therapy
Vetuximab (BV) has been used in combination with more than 80 different types of regimens, including cytotoxic chemotherapy and immunocheckpoint inhibitors. The combination of BV and immune checkpoint inhibitors is very promising. After confirming the efficacy of single-dose pablizumab in patients with recurrent Hodgkin's lymphoma and its superior activity compared with BV in this indication, some studies have explored the combination of BV with anti-PD1/PDL1 or anti-CTLA4 drugs. In patients with relapsed / refractory Hodgkin's lymphoma, BV combined with Navulizumab induced 82% of ORR, including 61% of CR.
T-DM1 has also explored the joint programme. Adding capecitabine to T-DM1 does not improve ORR, but it will lead to more adverse events. T-DM1 was also used in combination with patuzumab, and the results were compared. In the MARIANNE study, the overall survival of patients with advanced breast cancer treated with T-DM1 combined with patuzumab was similar, butQuality of lifeIt is better than patients who received trastuzumab and paclitaxel. T-DM1 is also used in combination with immune checkpoint inhibitors. The combination of T-DM1 and these drugs is supported by clinical and preclinical data.
Overall, these studies suggest that a carefully selected combination of ADC with other drugs may be superior to therapy based on uncoupled antibodies, both in terms of patient outcomes and safety. Safety is a key issue in combinatorial design, especially for patients with weakness or complications. Overlapping or accidental toxicity needs to be carefully monitored and adjusted according to the condition of each patient. Future studies will need to determine which patient subgroups benefit most from these combinations.
How to develop ADC in the future
ADC has established a solid position in the Cancer Pharmacopoeia. More than 1500 clinical studies of ADC are listed in clinicaltrials.gov, and more and more drugs are entering clinical trials (figure 4). It can be expected that the listing approval granted to ADC will be greatly diversified, as well as its indications in various diseases.
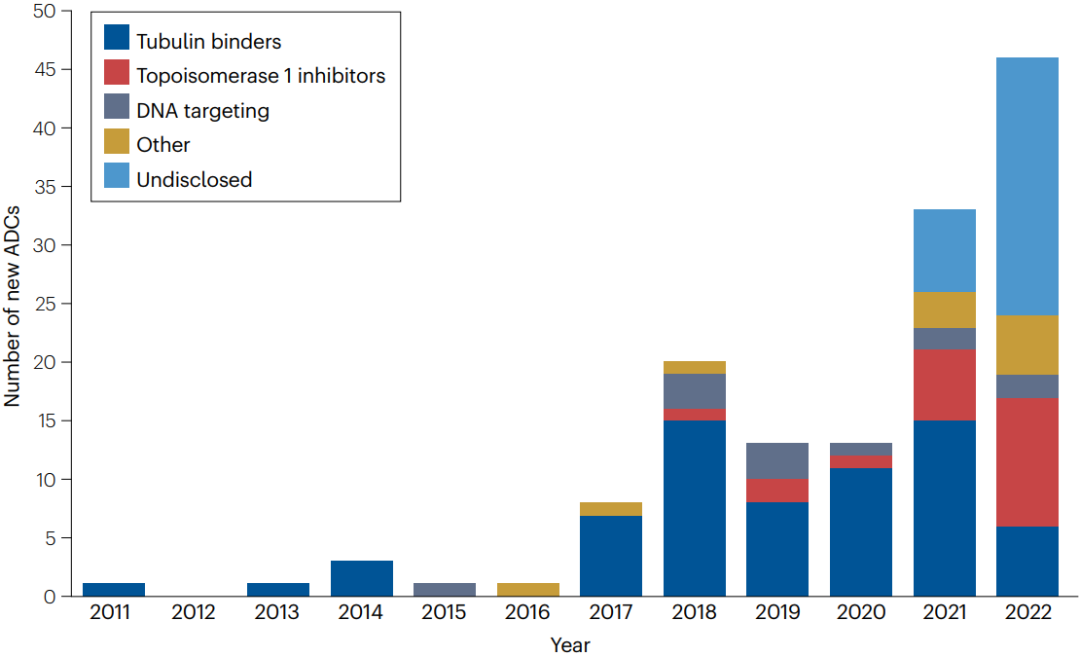
Figure 4 | the number of new ADC entering clinical trials between 2012 and 2022. In the past few years, the number of new ADC for clinical evaluation has increased rapidly. Since 2021, the proportion of ADC containing topoisomerase 1 inhibitors has increased. The proportion of undisclosed payloads is increasing (48% in 2022).
At present, almost half of the approved ADC is used for hematological malignant tumors. The difficulty in developing ADC in solid tumors may be due to specific characteristics, including poor diffusion, inherent resistance to cytotoxic drugs, and decreased mitotic scores. Due to the use of smaller forms or priority of intra-tumor activation with probody, better tumor permeability may enhance ADC activity in solid tumor indications. Some promising targets are currently being evaluated clinically for solid tumors (such as ROR1, HER3, CEACAM5, MET and NaPi2b), and a large number of tumor-associated antigens are currently evaluated as potential targets for ADC-mediated drug delivery.
The expected development of ADC will include new target antigens, payloads with new mechanisms of action, new linker techniques that can provide better therapeutic indicators, and new forms of antibodies and carriers.
1. Induce immunogenic cell death.
More and more studies are concerned with the immunostimulatory characteristics of ADC. In addition to the binding of the immune activator itself, such as in the immunoactive antibody coupling drug (iADC), ADC can also induce immunogenic cell death (immunogenic cell death, ICD), thus promoting anti-Tumor immunityReaction. The induction of ICD may be the reason for the effective combination of ADC with immune checkpoint inhibitors, especially in immune infiltration-rich diseases such as Hodgkin's lymphoma. Belantamab mafodotin induced ICD in vivo and activated dendritic cells in immunoreactive mouse model. ADC based on anti-HER2 anthracyclines also induces ICD and immunogenic memory. The ability of ADC payload to induce ICD may be different, and additional studies will help to determine its potential as an immune activator.
2. Targeting extracellular antigens
The initial model of anticancer ADC is based on the intracellular release of cytotoxic payloads, depending on internalization. The bystander effect and the ability of the payload to spread in the tumor depend on the physical and chemical properties and effectiveness of the payload. An exception to this rule is the use of non-internalized antibodies targeting extracellular components of the tumor microenvironment. For example, PNU-coupled antibodies targeting the splice domain of tenascin C induce complete remission in preclinical models; galectin-3 binding protein (LGALS3BP) preferentially secreted by tumor cells has been explored as extracellular ADC targets; other potential extracellular targets of ADC can be identified by high-throughput calculation.
Although the mechanism of these preparations is highly original, these new preparations face specific obstacles, including the relative expression of target antigens in normal and tumor tissues, the full release of payloads in the environment, and the effective infiltration of payloads in tumor cells. However, the clear approach to targeting extracellular targets of ADC is based on the concept that the extracellular release of diffusible bystander payloads may be an undervalued component of many mechanisms of ADC targeting solid tumors.
3. Elimination of immunosuppressive tumor environment
The third alternative for targeting tumor cells themselves and extracellular antigens is depletion of cell populations that affect treatment. Studies by Saha et al have shown that anti-CD45 ADC can receive allogeneic hematopoiesis.Stem cellBone marrow cell clearance (myeloablation) was performed successfully in transplanted mice, indicating the potential for patients to avoid whole body irradiation or exposure to strong alkylating agents. Recently, it has been reported that in the phase I/II study, myeloablative CD117 muscarine ADC is well tolerated. As we learn more about the role of immunosuppressive cells in the tumor microenvironment, ADC may be developed to deplete specific cell populations, such as regulatory T cells (Treg), type 2 macrophages, or myeloid-derived suppressor cells.
4. Beyond the standard ADC format
Although the development of ADC relies heavily on the targeting of standard monospecific antibodies, the industry is exploring alternatives such as probody coupling drugs (PDC) and biparatopic or bispecific ADC in order to enhance tumor specificity and reduce toxicity to healthy tissues. PDC is a masked prodrug that can be cleaved by proteolysis, which aims to provide therapeutic effect in tumor by taking advantage of the imbalance of tumor protease activity in tumor microenvironment. Probody masking peptides can prevent binding to targets in healthy tissues.
The rapid development of bispecific antibody technology provides more choices for the forms of antibodies used in ADC. Combining the payload on bispecific antibodies to produce bispecific ADC with improved specificity and / or internalization is a new research field, which is expected to overcome the existing limitations, such as endocytosis, toxicity and drug resistance of ADC. Nine bispecific ADC have entered the phase I clinical trial, and one has been discontinued (MEDI4276). Among them, 4 are biparatopic targeting two different epitopes on the same target, while 5 are targeting two different tumor-associated antigens.
Prospect
ADC has a significant effect on clinical oncology. Sales of several ADC are growing rapidly, with sales of three products exceeding 1 billion euros (BV, T-DM1 and T-DXd) in 2022, confirming the wide clinical use of ADC.
One of the main characteristics driving the development of ADC is that the therapeutic index of ADC is significantly higher than that of uncoupled (that is, traditional) cytotoxic drugs. However, this assumption has recently been questioned in terms of MTD values and the expansion of the treatment window. Therefore, to what extent ADC will replace the traditional cytotoxic chemotherapy remains to be explored.
The current limitations of ADC include its high cost and non-gastrointestinal administration. In addition, ADC is unlikely to be administered subcutaneously, while more and more nude antibodies are marketed through subcutaneous administration. However, ADC has established a strong position in anti-cancer drugs, and even if the development of these drugs is more complex than nude antibodies, the number of approved ADC is expected to increase significantly in the coming years to meet the growing number of common diseases andRare diseaseUnmet medical needs.

