-
Telephone:400-882-7987
-
wechat:15523376158wechat:15950040099

Since the launch of the first antibody-coupled drug (ADC) getozomib and Mylotarg, ADC drugs have stumbled through more than 20 years, and finally ushered in their own highlight moment. Especially in recent years, innovative ADC has been listed intensively, with detrituzumab (T-
Since the launch of the first antibody-coupled drug (ADC) getozomib and Mylotarg, ADC drugs have stumbled through more than 20 years, and finally ushered in their own highlight moment. Especially in recent years, innovative ADC has been listed intensively, and the ADC products represented by detrituzumab and Gosatuzumab have not only ignited the transaction and cooperation in the whole field, but also promoted the rapid growth of ADC market.
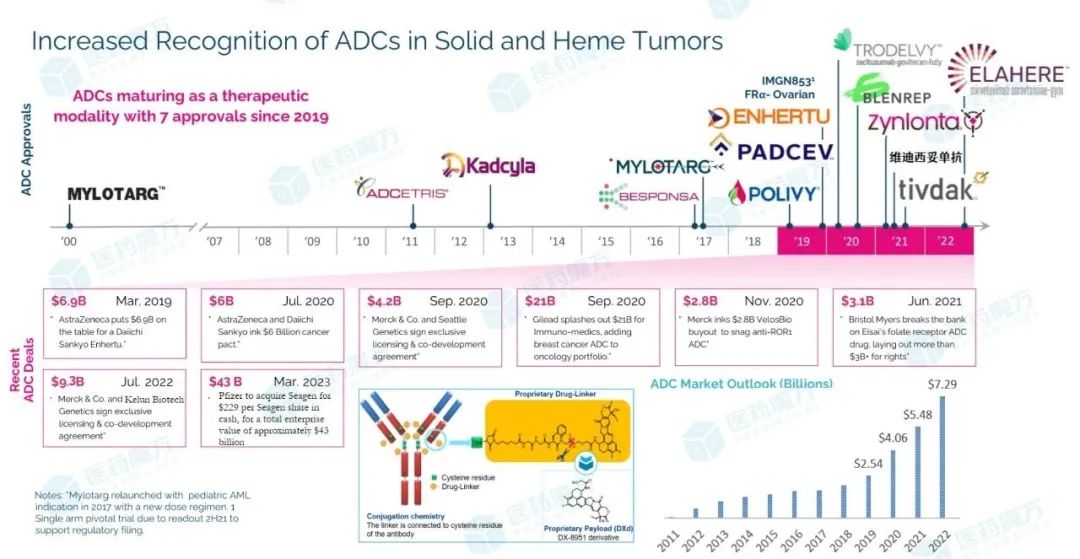
On February 24, the State Drug AdministrationAdministration and ManagementIt is officially announced that ®for injection has been approved for sale and is suitable for unresectable or metastatic HER2 positive adults who have previously received one or more anti-HER2 drugs.breast cancer. This also indicates that Detrozumab officially opened the prelude to domestic clinical reform.
As the hottest track nowadays, ADC attracts Pfizer to spend about $43 billion to strengthen its product layout. However, what is the future of ADC drugs and what clinical changes will it lead? In the wave of ADC, especially after the listing of Detrozumab, will the innovation direction of ADC change? In the future, how to take the road of breakthrough under China's innovative drug model? Are waiting for an answer.
The Origin of ADC: it begins with difference and ends with difference
At present, there are more than 650 active traditional ADC drugs in the world, only 14 products have been approved to go on the market, and nearly 200 products are still in different clinical research stages. Among them, HER2 ADC still has the highest proportion. Therefore, as an outstanding representative of HER2 ADC, the success of Detrozumab may change the development pattern and product strategy in the field of ADC. The most direct change has been reflected in the load selection of ADC drugs.
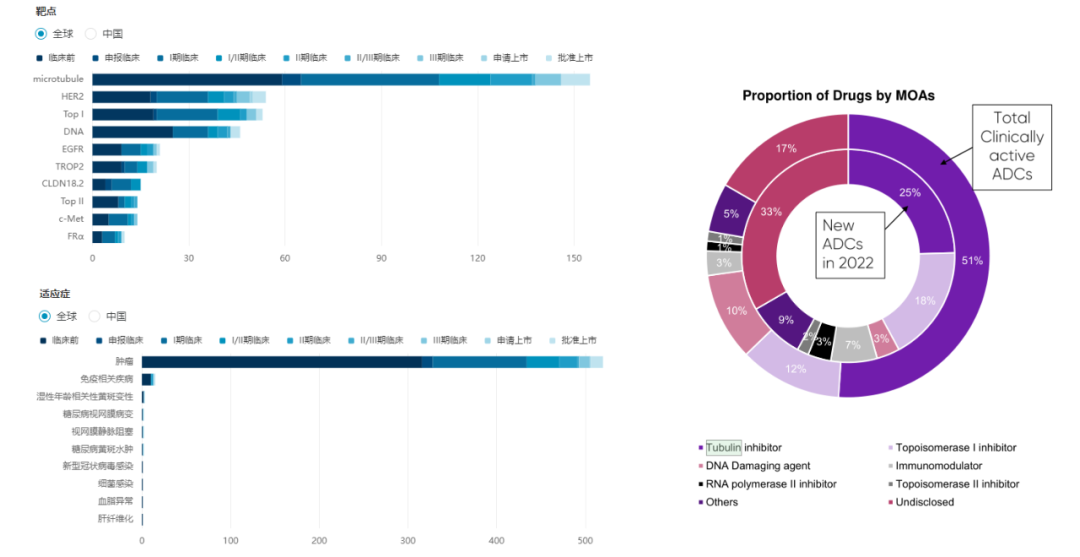
It is well known that ADC products are different from chemotherapy, targeted small molecules and monoclonal antibodies, but they are not celibate. ADC drugs are bridged by antibodies (Antibody) and cytotoxic drugs (Payloads, payload) through conjugates (Linker). In mechanism, it is generally believed that antibodies in ADC drugs play a "navigational" role by targeting antigens specifically expressed on the surface of tumor cells.PrecisionTo guide the drug to reach the focus, release cytotoxic drugs with efficient therapeutic effect inside the tumor, and finally specifically kill tumor cells.
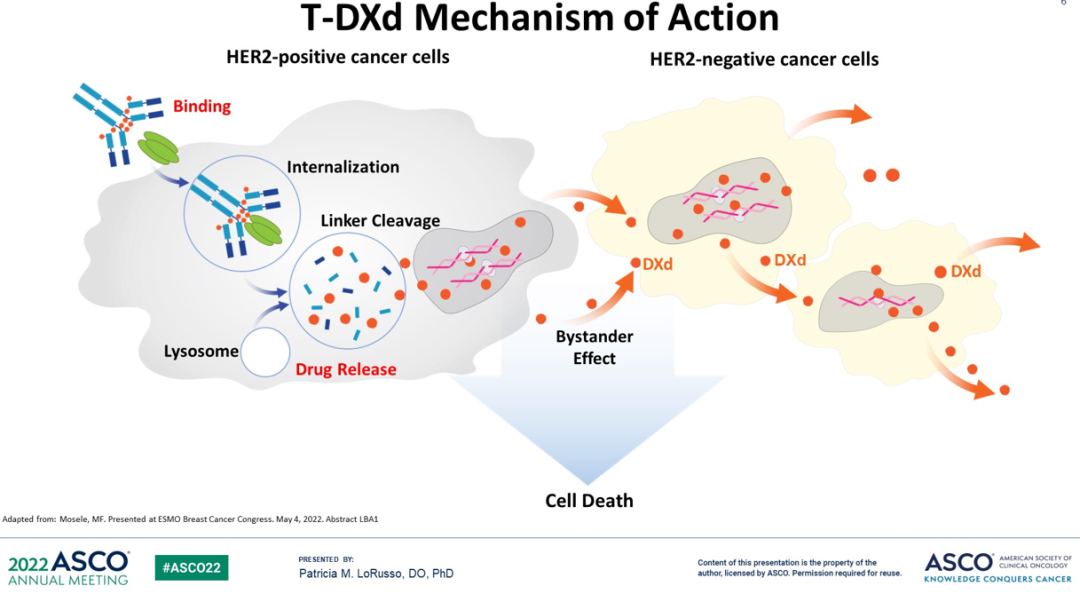
Source: 2022ASCO(LBA3 by Shanu Modi[1])
Taking detrozumab as an example, the antibody (trastuzumab analogue) in the drug binds to the antigen HER2 expressed on the surface of tumor cells, fuses with lysosome after endocytosis, and cleaves Linker by lysosomal enzyme degradation to release cytotoxic drugs (DXd). At the same time, because DXd has cell permeability, it can also kill nearby HER2-negative tumor cells (bystander effect).
Of course, not all ADC is through the breakable Linker link, the listed nmetrozumab and delisted Blenrep is the use of non-breakable Linker. Similarly, not all loads have cellular permeability, for example, the toxin (lysine-MCC-DM1) degraded by nmetrozumab cannot efficiently permeate the cell membrane. Therefore, there are some differences in the mechanism, but on the whole follow the above mechanism process. Generally speaking, the mechanism of action of ADC drugs in the industry has been relatively unified.ConsensusBut the perception of ADC drugs may be debatable.
First of all, based on the understanding of the mechanism of action of ADC, the clinical value of ADC is generally attributed to cytotoxic drugs (payloads), even called "delivery tools", including review experts have openly stated that ADC is a chemotherapeutic drug. Of course, this understanding is reasonable, but it is difficult to say that different ADC drugs are completely correct in different clinical application scenarios.
Still take detrituzumab as an example. Detrozumab uses trazumab analogs to target HER2, but in the DESTINY-Breast04 study, Detrozumab has been used in patients with breast cancer with low expression of Her2. Obviously, this study would not have been carried out as a delivery tool, and there would not be a new treatment for breast cancer with low expression of Her2. In addition, the DESTINY-Breast09 study is a clear example, in which the standard therapy of trostuzumab and trostuzumab opens a first-line head-to-head study for the treatment of HER2-positive breast cancer. There is no doubt about the clinical value of trastuzumab combined with chemotherapy in HER2 positive breast cancer patients. In the past 20 years, few drugs have been able to win in the head-to-head research, which shows that the first and third communists really have different thoughts on Detrozumab.
In fact, from the background of clinical treatment, there is also a hypothesis that ADC is different from chemotherapy. For newly diagnosed HER2-positive breast cancer patients who have not been treated with trastuzumab and / or patuzumab, trostuzumab containing trostuzumab analogues will not only act as a "delivery tool", but may have a superimposed effect on clinical outcomes. In addition, the targeting effect of ADC drugs can avoid or reduce the safety problems caused by systemic exposure of chemotherapeutic drugs, and increase the tolerance of chemotherapeutic drugs. Therefore, in terms of efficacy and safety, ADC drugs are also different from the clinical treatment mode of monoclonal antibody plus chemotherapy.
Secondly, ADC drugs are still being improved and developed. In recent years, the load has also undergone a variety of changes, except for cytotoxic drugs.ImmunityStimulating molecules, targeting small molecules and protein degradation molecules are also the exploration direction of ADC drugs, so it is inevitable to define ADC as chemotherapeutic drugs.
In short, at the beginning of its birth, ADC is different from the drug design of chemotherapy, targeting small molecules and antibodies, which also determines the different clinical mechanisms of various drugs. At the same time, with the development of ADC technology and the change of drug cognition, more differentiated products will continue to emerge, or will continue to bring about a change in the paradigm of clinical treatment like detrazumab.
ADC change: the re-evolution of the treatment pattern
Reviewing the 100-year development history of tumor therapy, it has gone through three revolutions, and also led the three evolution of the era of chemotherapy, targeted therapy and immunotherapy of clinical treatment. It can even be said that the change of drugs promotes the revolutionary evolution of each tumor treatment, and also makes each revolutionary process have the characteristics of drugs.
The era of chemotherapy broke the dilemma of only surgery and radiotherapy as clinical treatment, and opened the exploration of drug treatment of cancer. Chemotherapeutic drugs represented by paclitaxel, docetaxel, irinotecan and platinum have a wide range of indications, opening up the clinical practice of combined surgery and / or radiotherapy. However, the safety caused by systemic exposure of chemotherapy and radiotherapy affects the quality of life of tumor patients. In the era of targeted therapy, most drugs achieve specific targeting and reduce systemic toxicity. Rituximab and imatinib highlight the clinical strength as the representatives of antibody and small molecule targeted drugs respectively. It opens the clinical practice of precision medicine, but unfortunately, precise targeting design also limits the indication space of drugs. In the era of immunotherapy, PD-1/L1 antibody drugs not only let us appreciate the advantages of immunotherapy, but also let us see the pan-tumor potential of immunotherapy again, but it also has the defect of congenital drug resistance or insufficient response.
In the drug era of ADC, what changes will take place in the mode of tumor treatment? How will the pattern of tumor treatment evolve?
At present, I am afraid it is not possible to fully determine the ultimate ict of ADC drugs on the evolution of tumor treatment, after all, innovative products are still emerging. However, in terms of the clinical manifestations of the current products, we can see that ADC drugs, like chemotherapy and immunotherapy, have successively filled the clinical treatment gaps or reshaped the treatment standards, and continue to expand the treatment depth of the disease. At the same time, we continue to achieve clinical breakthroughs ass the tumor field, highlighting the breadth of treatment of ADC drugs, and this potential seems to be perfectly explained by the mechanism of ADC drugs. The systemic exposure toxicity of chemotherapeutic drugs is reduced by specific targeting, and the carrying cytotoxic drugs help it to have pan-tumor qualification.
Obviously, most of the innovative ADC therapy products on the market have this potential, and the ADC products targeting HER2 and TROP2 are more eye-catching. In particular, the head-to-head study of dituzumab in patients with HER2-positive breast cancer has a better clinical benefit than T-DM1.[2]There is no doubt that it will not only become a new treatment standard, but also an unavoidable challenge for similar products. Throughout the clinical research layout of Detrozumab, it is not difficult to see that Detrozumab includes end-line treatment, second-line treatment, first-line treatment, adjuvant and neoadjuvant therapy for HER2-positive breast cancer, covering the management of the whole course of the disease from early to late stages, demonstrating its continuous effect on the depth of treatment.
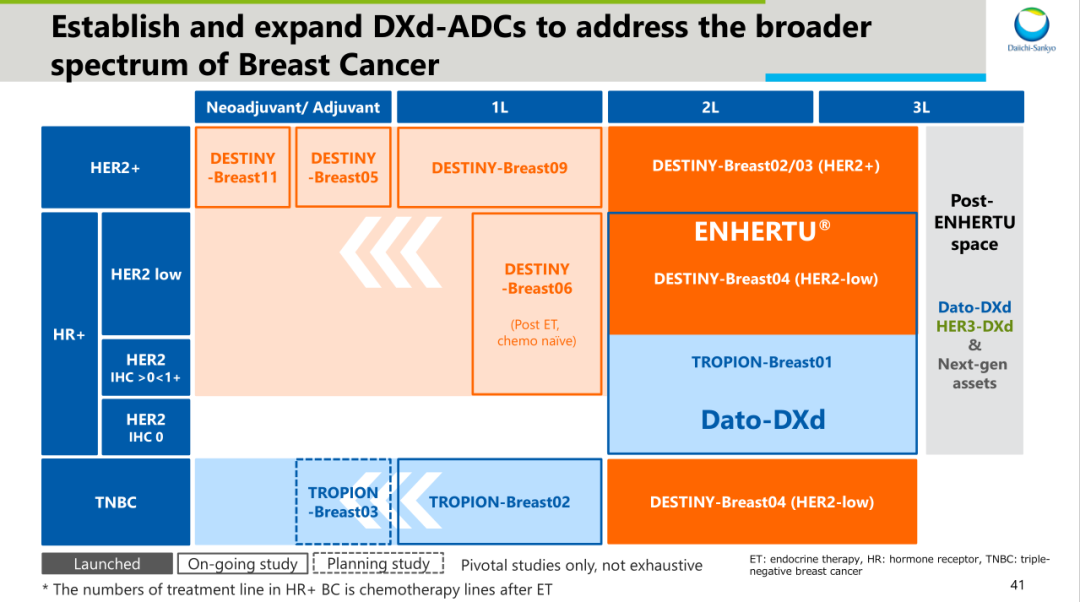
Source: information on the first three R & D days[3]
In addition, the pan-tumor potential of detrituzumab is evidence of the breadth of drug treatment. All over the world, Detrozumab has been approved as HER2 positive breast cancer, HER2 low expression breast cancer and HER2 positive breast cancer.Gastric cancerAnd gastroesophageal junction carcinoma and HER2 mutant non-small cells.lung cancerEspecially in breast cancer with low expression of HER2 and non-small cell lung cancer with HER2 mutation, Detrozumab is the first approved targeted drug in the world.
Recently, the II DESTINY-PanTumor02 study of detrituzumab has reached the main end point.[4]The study included biliary tract cancer, bladder cancer, cervical cancer, endometrial cancer,Ovarian cancer、Pancreatic cancerAs well as advanced patients in cancer areas such as other rare cancers. The same is true of TROP2 ADC, which not only subverts the pattern of advanced TNBC treatment, but also achieves a milestone in urothelial cancer and HR-positive breast cancer. In addition, the exploration of non-small cell lung cancer has also entered the late clinical stage.
HER3 ADC is also found in non-small cell lung cancer, breast cancer,colorectal cancerAnd triple negative breast cancer and other tumor areas to carry out extensive exploration. Among them, the data released by HER3-DXd (patritumab deruxtecan) at the just-concluded 2023 Japanese Society of Clinical Oncology (JSMO2023) show that[5]For locally advanced or metastatic EGFR mutations who have received multiline therapyNSCLCIn patients, the ORR assessed by the blind independent center review (BICR) of HER3-DXd reached 40.2%, and the median overall survival time was close to 16 months, especially for patients who had received third-generation EGFR TKI and platinum treatment, the median overall survival time was 16.2 months, which once again demonstrated the clinical transformation potential of ADC drugs.
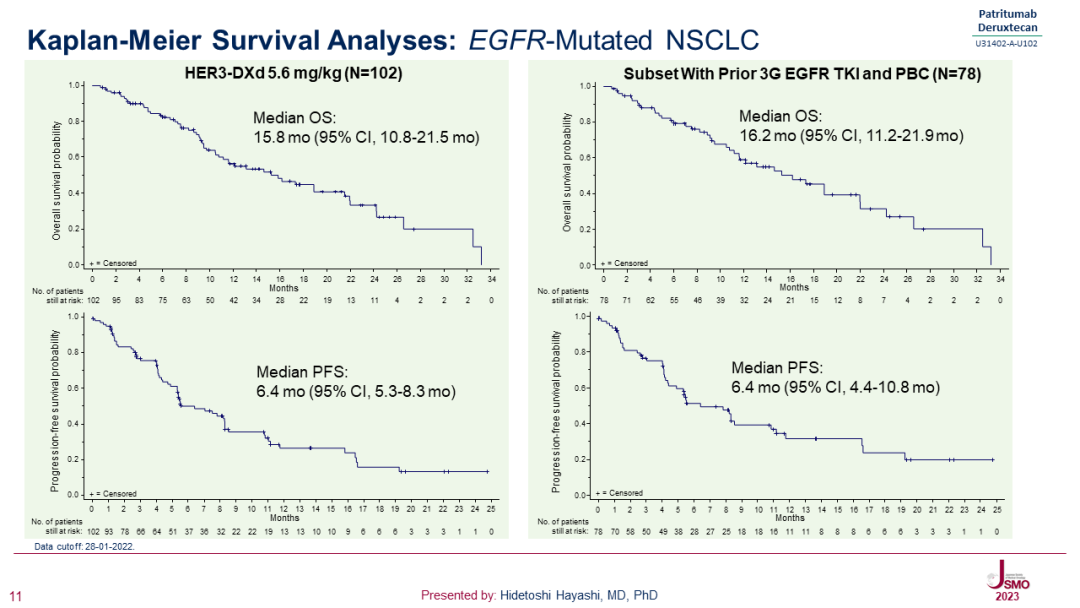
Source:JSMO2023(Presented by: Hidetoshi Hayashi, MD, PhD)
It can be said that innovative ADC products are changing clinical treatment norms with unstoppable boldness, and at the same time affecting the development pattern and strategy of ADC products. Especially after the implementation of the guiding principles of Clinical value-oriented Clinical Research and Development of antineoplastic drugs, the guiding opinions on the standard of control drugs in clinical research are also given, and the principle of guiding clinical value is clearly put forward. innovative drugs are required to reflect clinical advantages over control drugs. Therefore, after the innovative ADC products achieve clinical breakthrough and transform into standard treatment, how to evolve the future clinical demand and how to develop ADC drugs will become the challenges that the next generation of ADC products need to face.
The Future of ADC: where to go?
In 1913, the famous German chemist Paul Ehrlich (Paul Ehrlich) put forward the concept of "magic bullet"[6]It is considered to be the earliest description of ADC drugs. Year 2000FDAMylotarg, the first ADC drug, was approved to be put on the market, but the ADC drug was withdrawn from the market because of its insignificant efficacy and off-target toxicity, making ADC fall into the abyss of darkness again.
Since then, with the development of antibody drugs, the progress of coupling technology and the continuous improvement of the concept of ADC and other factors, after several generations of ADC drugs exploration and verification, finally ushered in a glorious moment belonging to ADC. The original "magic bullet" has also been upgraded to a "magic missile".
In fact, the targeting effect of ADC drugs expands the treatment window of traditional chemotherapy, making it possible for ADC to carry a higher cytotoxic load. The load SN-38 carried by Gosatuzumab is more than 100 times higher than that of irinotecan.[7], and the DXD carried by Detrozumab is about 9 times higher than that of SN-38.[8]. At the same time, due to the targeting effect, it can be released around the tumor cells in high concentration, and even exert the bystander effect to kill the adjacent tumor cells, so as to achieve the synergistic effect of "1-1 > 2".
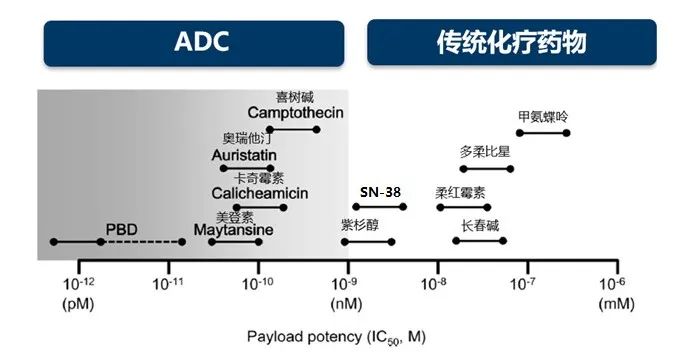
All kinds of payloads approximate cytotoxic range.[9]
However, how to balance efficacy and safety has always been a challenge in the development of ADC drugs, and it is also a bottleneck restricting the development of ADC drugs.
In the face of the continuous clinical impact of Detrozumab, the development of ADC drugs with safety potential in theory was stopped because of the influence of competition pattern. Blenrep, the first ADC therapy targeting BCMA, became the second ADC drug to experience withdrawal because of its poor efficacy in confirmatory clinical studies. These phenomena prove once again that the iterative development of innovative drugs has never been a smooth road.
Therefore, pharmaceutical companies that have the courage and courage to face the challenges of the next generation of ADC drug development may be qualified to decide what ADC will look like in the future. After 10 years of ingenuity to build the DXd technology platform, now the success has not only created a successful example of Detrozumab, but also completed the verification of the DXd technology platform, but also attracted the attention of tumor advantage MNC like AstraZeneca, and jointly developed and promoted two DXd technology products including Detrozumab. After Detrozumab, the first three Communists have put forward the idea of "next-generation/new-concept ADCs", which makes the industry full of expectations.
Of course, in order to break through the shackles of the development of ADC drugs, different companies are trying to give their own innovative answers. Generally speaking, the pioneering exploration is mainly based on the composition of ADC drugs. In the part of antibodies, the mainstream theory is still to explore tumor-specific highly expressed antigens to avoid targeting normal cells, but some companies are trying the concept of Probody to achieve selectivity through tumor-specific targeted activation of antibodies, which is expected to improve the potential of ADC drugs and expand the choice of antigens.
Linker and load mainly focus on solving the toxicity problems caused by early release in the cycle, including the use of site-specific coupling, unnatural amino acid coupling, click chemistry and other techniques to form a more stable covalent coupling to avoid the separation of antibodies from Linker. Secondly, the load also has the design attempt of Probody concept, by virtue of β-glucuronidase sensitive junction, fibroblast activated protease cleavage and so on to achieve the specific release of tumor environment. In addition, the load of innovative new mechanisms such as immune activation and protein degradation is also a new territory to explore, and there is a dual-mechanism ADC design concept.
At the same time, the understanding and understanding of ADC are also improving, and it is not impossible for ADC drug load to be released outside the tumor cells. However, these innovative explorations are still in the early stage of research, and more technological innovations and concepts of the ADC track in the future need to be proved by clinical concepts.

